-40%
Dental Scaler Tip P1 Perio Compatible with EMS Scaler %INX
$ 3.35
- Description
- Size Guide
Description
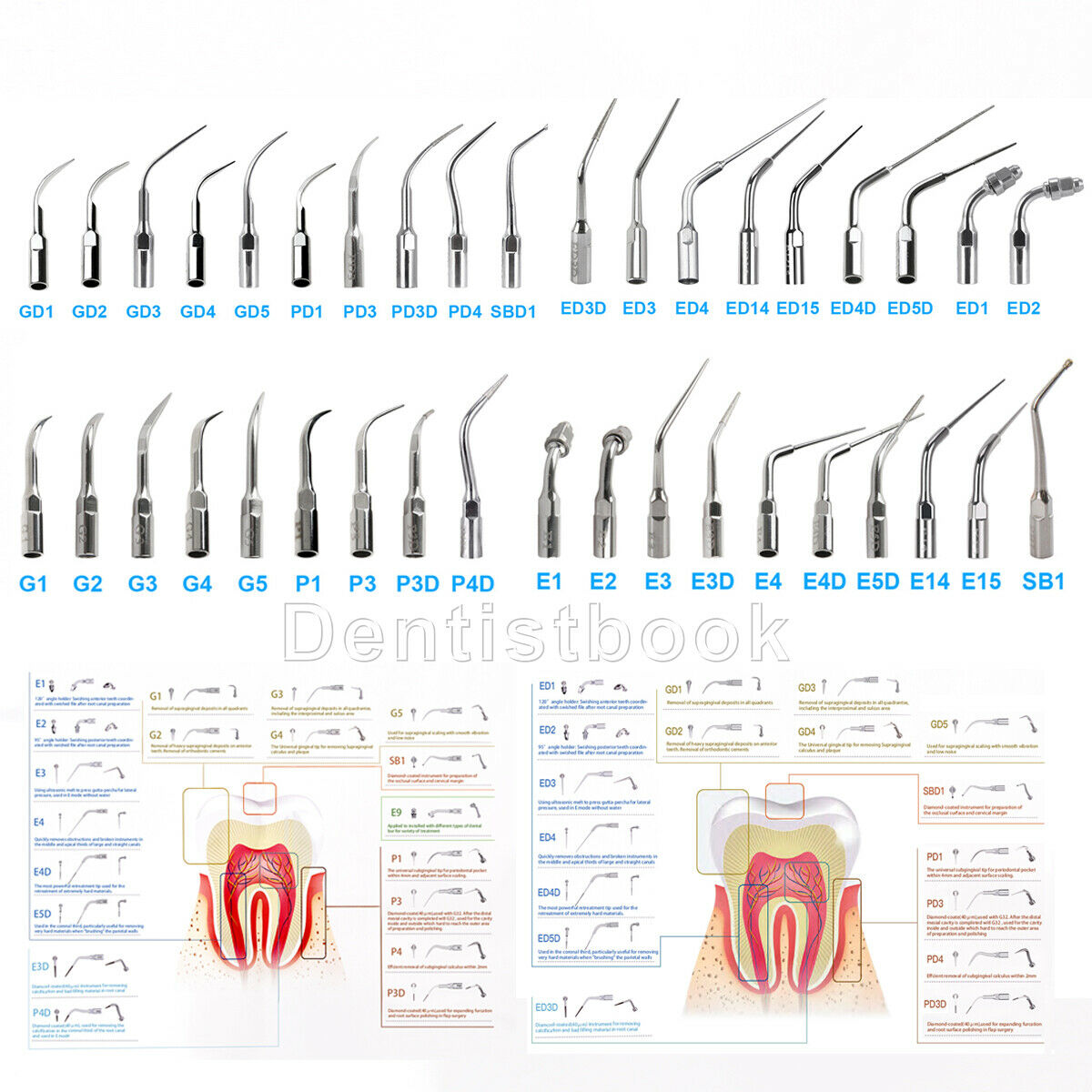
Dental Ultrasonic Scaler Handpiece with P1 Scaler Tip fit EMS SKYSEAFeatures:
P1:
Remove subgingival calculus.
Payment:
1.We accept Paypal only.
2.Payment should be made within 7 days of auctions close.
Shipping:
1. The item will be dispatched within 24 hours after payment received in full and cleared.
2. Please make sure your address match the paypal address before you pay.
3. Import duties, taxes and charges are not included in the item price or shipping charges. These charges are the buyer’s responsibility.
4. Please check your country’s customs office to determine what these additional costs will be prior to bidding/buying.
Feedback:
1. Please leave us a
Positive Feedback
if the product is good.
2. Your feedback is our motivation to develop our business and serve you better.
Return & Refund:
Return within 60 days is accepted, though the item in good condition.
Important Notice For U.S.A Buyer
The following FDA Disclaimer is required for all eBay listing in healthcare category:
The sale of this item may be subject to regulation by the U.S. Food & Drug Administration and state and local regulatory agencies.You can bid on this item if you are an authorized purchaser. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health: .
( )
510(K) Number: K163414
Regulation Number: 872.4850
Product Code: ELC